One of the main challenges in cancer chemotherapy is how to selectively kill tumour cells while leaving healthy cells alive. Researchers at the Indian Institute of Science Education and Research (IISER) Pune have come up with a novel approach where they use an artificially constructed ion channel and certain biochemical peculiarities of cancer cells to induce cell death in a highly targeted manner.
A recent study led by Pinaki Talukdar, Associate Professor at the Indian Institute of Science Education and Research (IISER) Pune presents a novel approach towards targeting cancer cells. The researchers have explored certain organic molecules which can form pores in the membrane of cancer cells, thereby inducing cell death. The study has potential applications in the anticancer drug discovery process.
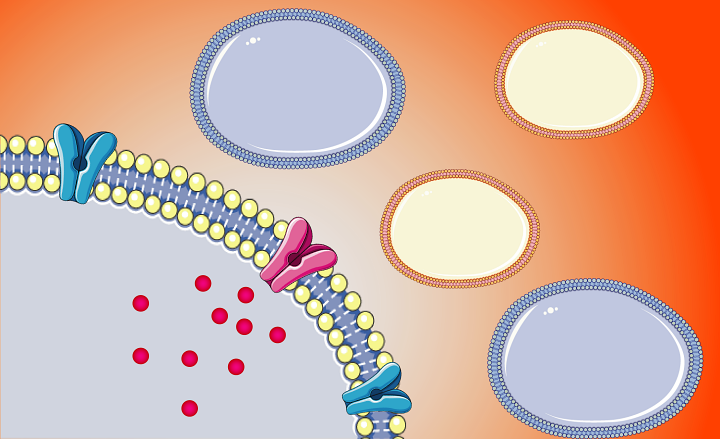
Cell membranes have become hotspots of research in recent times since they serve as entry points into the cell. Ion channels are large protein structures embedded in the cell membranes that are involved in the passage of ions like sodium, potassium or chloride. They play a crucial role in gatekeeping and maintaining cellular homeostasis. Talukdar and his team studied the structure of these ion channels and tried to mimic them artificially using small organic molecules that can be synthesized in the laboratory.
The researchers found that the small molecules they synthesized could interact among themselves when placed within lipid bilayers like those found in cell membranes to form bigger structures called “supramolecular nanotubes”. They also found that these structures can act as ion channels and transport sodium, potassium, and chloride ions across the membrane.
When the movement of sodium, potassium, or chloride ions across the cell membrane gets disturbed, it can result in cell death through a process called apoptosis. The team studied whether the synthesized molecules could perturb ion transport in living cells. After a series of experiments in cells grown in the lab, they succeeded in inducing apoptosis in cells incubated with the novel supramolecular structure. “We then asked the question, how can we apply our system selectively into cancer cells?” says Talukdar.
Cancer cells have increased levels of glutathione, an antioxidant. Glutathione protects these cells from free radicals, reactive oxygen species, and many commonly used anticancer drugs. The team decided to take advantage of this feature. They connected a chemical group to the active molecule which prevents it from forming the supramolecular nanotubes. However, this chemical group gets cleaved in the presence of high glutathione concentrations in cancer cells, allowing the system to form an active ion channel in the cancer cell membrane. This, in turn, leads to a disruption of the ionic balance, an increase in reactive oxygen species (ROS), and a decrease in glutathione levels, ultimately resulting in cell death.
“One of the most important strategies for using ion transporters as anti-cancer agents is to make them only disrupt cancer cells and not affect normal cells,” says Philip Gale, University of Sydney, who was not involved in the study, pointing out that the current study’s approach relies on a specific feature of cancer cells — high glutathione levels. “This is a very selective way of killing cancer cells and may lead to new treatments for cancer,” he adds.
The team compared the system’s efficacy in killing cancer cells with the anticancer drug doxorubicin, and noted similar results. Dipak Datta, Principal Scientist at Central Drug Research Institute (CDRI), Lucknow, and Abhipsa Jain, a JRF in Datta’s lab, say, “Such studies provide excellent approaches to employ the application of synthetic ion transport systems to sensitize tumours to cytotoxic effects of anticancer agents and present a promising therapeutic approach for the treatment of cancer.”
“Designing such compounds and executing them properly in cells was a big challenge. We had to face a lot of hardships and it took almost four years to reach this,” says Javid Ahmad Malla who is the first author of the paper. Talukdar adds that their future work will involve studying this system using animal models.