In a country where more than 80% of medical devices are imported, IIT Bombay researchers have developed India’s first biodegradable bone screw. The screw is made of a polymer-based biomaterial which contains Magnesium Oxide nanoparticles and silk fibres, and its mechanical strength can be tuned to match the target tissue. As tested in rodents, the screw decomposes reasonably fast and is completely compatible inside the body.
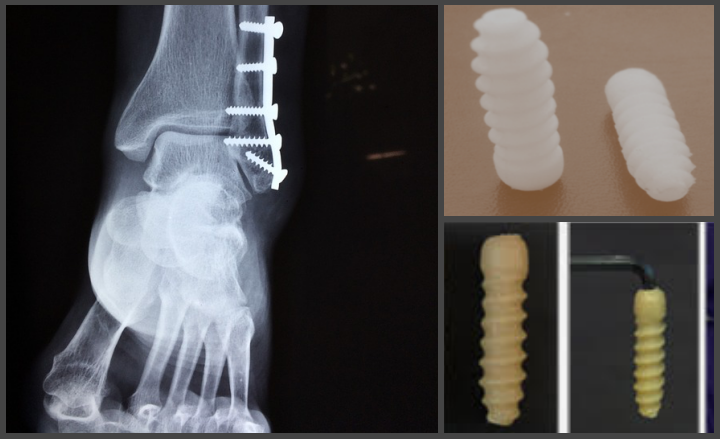
How many of you were fitted with those painful metallic implants after you fractured a limb? Orthopaedic surgeons, like carpenters, use metal screws, but for fixing bone/tissue fractures rather than furniture. However, studies suggest that metal implants hamper bone growth and development, especially in children. A team of researchers led by Rohit Srivastava and Jayesh Bellare at the Indian Institute of Technology (IIT) Bombay have recently developed a bone screw made out of a polymer composite biomaterial, which naturally degrades in the body over time.
“We consulted several orthopaedic surgeons to analyse the unmet clinical needs of orthopaedic practices in India,” says Ajay Suryavanshi, the first author of the study. While biodegradable polymer-based screws made of Poly Lactic Acid (PLLA) are used commonly these days, Indian companies don’t yet possess the license to manufacture them. Hence, they need to be imported, which adds to treatment costs, often making them unaffordable. The current study, which uses polycaprolactone instead of PLLA as the main component, addresses this problem.
“This is the first step towards getting indigenous biodegradable screws approved in India,” says Srivastava.
Interestingly, the researchers found that the addition of fillers like Magnesium Oxide (MgO) nanoparticles and short silk fibres significantly improved the polymer’s mechanical strength and helped it degrade faster. After experimenting with various concentrations of the main components, the team hit upon an optimum combination which had enough tensile strength to reconstruct soft-tissues.
Next, using cell-culture based studies, the researchers found that the new biomaterial could easily attach to human osteoblast-like cells (a bone-derived cell type) and enhance cell proliferation. It also degraded at a faster rate over the period of a year compared to standard PLLA screws. Success with the in vitro studies led the researchers to fabricate a screw for Anterior Cruciate Ligament (ACL) reconstruction, a type of surgical tissue graft replacement in the knees.

Upon testing the screw by implanting it in a rat model, the researchers were happy to note that it didn’t adversely affect any vital organs, or lead to abnormal blood readings, suggesting a high level of biocompatibility. However, the screw did cause an initial inflammatory response, which subsided gradually. The team ended the study by successfully performing an ACL reconstruction surgery using the screw in a pig’s knee joint.
As both Suryavanshi and Kunal Khanna, another lead author of the study, agree, the work had its challenges. “Due to lack of infrastructure support, moulding polycaprolactone along with the evenly distributed fillers was challenging,” Suryavanshi says.
“Although a more detailed degradation kinetics study is required, this is a highly impactful study, especially in India’s medical devices sector,” says Bikramjit Basu, Professor at Indian Institute of Science, Bengaluru, who wasn’t associated with this study.” Dr Gautam Shetty, Orthopaedic Surgeon and Head of Research, QI Spine Clinic, India, also lauded this study. “The team intends to replace some of the metallic implants used commonly in orthopaedic surgery in India with inexpensive indigenous bioresorbable implants,” says Shetty, “This is an ambitious and commendable goal!”
“We have completed preliminary observations,” says Srivastava, discussing the potential applications of this technology. “We have the right combination to start the next set of studies involving larger animals and finally into Human Clinical Trials after GMP grade manufacturing,” he adds. However, Bellare is more cautiously optimistic. “Even though they are willing, the Indian orthopaedic medical devices industries will take some time to add polymer manufacturing units in their factories to build such products,” he says.
This work has received the HTAC-ICMR Business Development Award from the Indian Council of Medical Research (ICMR) and FICCI in collaboration with IC2 Institute Business Incubator, University of Texas at Austin, USA, amongst other prestigious awards.
Did you enjoy this article? Please let us know in the comments below.