Cholesterol plays a key role in the recently discovered mechanism.
Immune cells perform their protective function by engulfing disease-causing pathogens and destroying them. Researchers from the Tata Institute of Fundamental Research (TIFR), Mumbai, have unraveled an important step in this process, and discovered that cholesterol is an essential player in the mechanism. Their findings, published in the February 11 issue of Cell, could be the key to understanding immune responses better and developing treatments against infectious diseases.
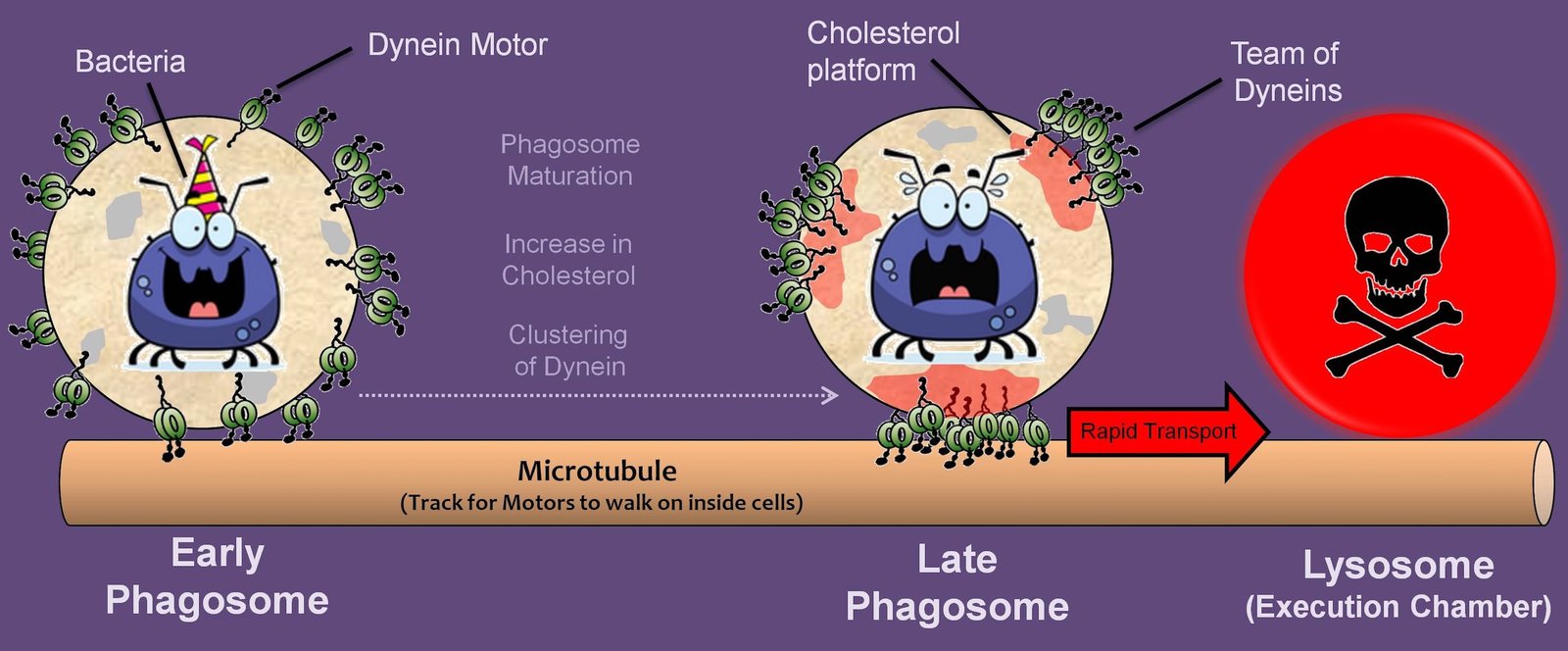
The tiny cells that make up our bodies are continually abuzz with activity. Their long list of functions includes synthesis and transportation of proteins and cellular bodies to different parts of the cell, segregation and replication of genetic material, and energy production. Many of these processes require molecular motors to ferry cellular components across the intracellular highways. Motor proteins such as dynein and kinesin generate minute forces for the transport of cellular cargo. In addition, these motor teams play a critical role in our immune response to pathogens.
“Pathogens, such as bacteria and parasites, are first enclosed by the immune cells in a compartment called the ‘phagosome’. The phagosomes are then transported around by the molecular motor teams and are ultimately taken to an execution chamber called the lysosome, where the pathogen is killed,” explained Roop Mallik, the principal investigator of the study. Interestingly, early phagosomes move in a bidirectional back-and-forth manner near the cell periphery, and it is only after maturation that there is a switch, that causes the phagosomes to move in an almost unidirectional manner towards the cell interior for degradation.
How are the motor teams assembled to bring about phagosomal motion? What underlies the bidirectional-to-unidirectional motion-switch of the phagosome? To investigate these questions, Mallik and his team used artificially generated phagosomes from latex beads and studied phagosomal transport inside cells. They found that the phagosomal membrane has cholesterol-rich regions, which facilitate clustering of dynein motor proteins at those sites. The clustering grows progressively as the phagosome matures. The cholesterol-induced team of dynein motors can then generate large cooperative forces to transport the matured phagosome. These forces enable the motion-switch and drive the rapid unidirectional transport of late phagosomes to the lysosomes for degradation. “We have found that the transport of pathogens to lysosomes is achieved by the physical clustering of many nanoscale dynein motors,” said Mallik. “Cholesterol — usually a much hated molecule — shows a kinder face by helping to kill the bugs that infect us,” he added.
Explaining the significance of this finding, he said that many pathogens infect us because they avoid being taken to the lysosomal execution chamber and that their research reveals, for the first time, the basic mechanism by which phagosomes are taken away for degradation. “Now that we understand this, we can ask how certain pathogens bypass this execution mechanism. We have already taken a step in this direction in the recent paper. We show that a lipid molecule from Leishmania donovani, the parasite that causes kala-azar, disrupts the clustering of dynein and thus prevents transport of the parasite-containing phagosome to its execution,” said Mallik. This enables the parasite to spread the infection by surviving and multiplying inside the immune cells of our liver and spleen. Aided by these results, Mallik and his team now plan to test various molecules and identify the one(s) that can disable the kala-azar lipid, and restore the clustering of dynein. Such a molecule can be a potential drug candidate for the disease.
Visceral leishmaniasis or kala-azar, is a deadly disease endemic in many tropical countries, including India. Pathogens causing various other diseases like tuberculosis and typhoid also escape the transfer to the execution chamber. Their avoidance mechanism may be similar to that of the kala-azar parasite. Research done by Mallik’s lab is a step in the direction of learning more about these infections, and potentially developing effective cures.