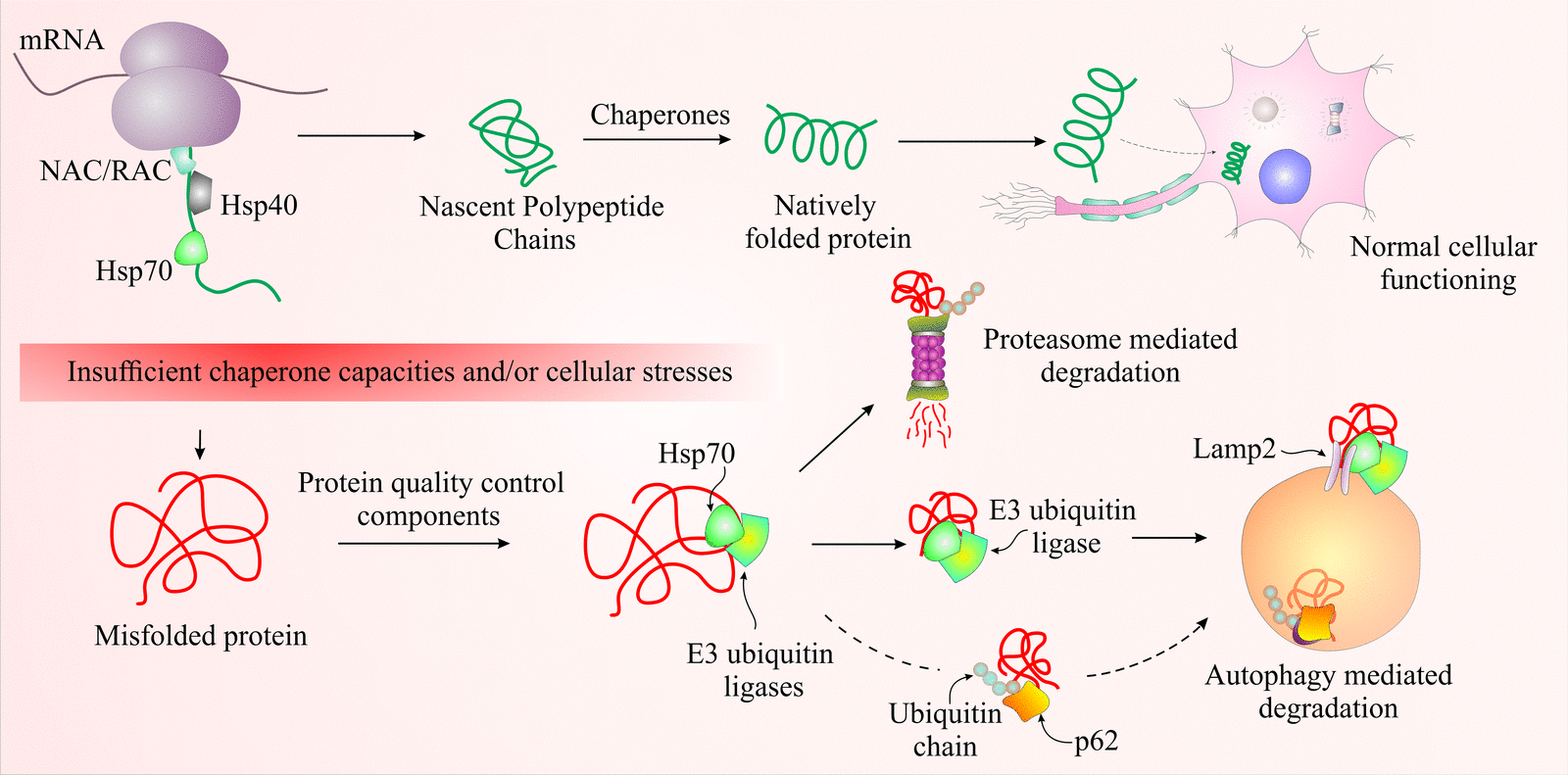
Researchers at the Indian Institute of Technology, Jodhpur have identified that the mahogunin ring finger protein – 1 (MGRN1) promotes elimination of misfolded proteins; thus alleviating the cytotoxic risk posed by damaged proteins. The degradation of altered or misfolded proteins is a chief intracellular function to maintain homeostasis and survival. Unfortunately, this essential function is often hampered resulting in a diseased state. Misfolded proteins are observed in the pathology of a number of incurable neurodegenerative diseases and protein conformation disorders. They cause cellular toxicity and may eventually result in cell death. In living cells, majority of misfolded proteins are eliminated via two proteolytic systems i.e. autophagy lysosomes and the ubiquitin proteasome system. Researchers at IIT Jodhpur have conclusively demonstrated that MGRN1 is involved in the elimination of misfolded proteins via selective autophagy pathway.
Autophagy essentially means ‘self-degradation’, an activity performed by every cell in order to eliminate waste material. Chaperone-mediated autophagy (CMA) is a specialised pathway that is involved in the selective destruction of misfolded proteins in cells. In this pathway misfolded proteins having a particular motif i.e. KFERQ or a biochemically related motif are targeted by a cytosolic chaperone hsc70 for further destruction. A chaperone is a protein that helps with folding and assembly of nascent polypeptide chains. Amit Mishra et al found that MGRN1 is involved in the degradation of misfolded proteins via association with Hsp70 molecular chaperone. Although the mechanism for specific targeting of abnormal proteins by Hsp70 yet remains elusive, the involvement of MGRN1 has been demonstrated.
The IIT Jodhpur team experimentally proved that MGRN1 associates with chaperone-linked misfolded protein inclusion bodies and suppresses cell death generated by abnormal proteins. Loss of MGRN1 function develops age-dependent spongiform neurodegeneration (formation of vacuoles in neuronal cells) in mice. Exposing the cells to various stress inducing agents showed elevated endogenous levels of MGRN1, this result suggests it’s active molecular role in cellular quality control pathway. Further Mishra’s team observed a novel interaction between MGRN1 and Hsp70 molecular chaperone. Cumulative function of Hsp70 and MGRN1 additively enhances cytoprotection against cell death due to oxidative and endoplasmic reticulum stress. This study has, for the very first time, elaborated the quality control function of MGRN1 E3 ubiquitin ligase.
Insufficient or retarded abnormal protein elimination in neuronal cells leads to various neurodegenerative diseases such as Alzheimer’s, Huntington, and Parkinson’s, Amyotrophic lateral sclerosis and polyglutamine diseases. This reinforces the urgency for a therapeutic strategy against abnormal proteins. Probing further into this research, in order to decipher the entire process of misfolded protein elimination and most importantly of identification of misfolded proteins in the cell, could allow researchers in the future to modify the pathway and increase its efficiency. Another important consideration would be the implications of over-expressing MGRN1; apart from its “clearing” ability any possible negative impacts must also be studied. Overall this study suggests that in the near future a detailed understanding of the molecular mechanism of MGRN1 and efficient upregulation of its quality control function may generate a potential therapeutic opportunity with profound implications for neurodegeneration and aging.
Further reading:
Chhangani D, Mishra A. Mahogunin ring finger‑1 (MGRN1) Suppresses Chaperone-Associated Misfolded Protein Aggregation and Toxicity. Sci Rep. 2013;3:1972. doi: 10.1038/srep01972.