As a nation, we have made enormous progress in medical science and technology since independence, resulting in increased longevity and quality of life of our citizens. At the same time, clinical research in India has been plagued time and again by ethical controversies. In this next article in our series on research ethics, we explore the available guidelines for maintaining high ethical standards during clinical trials in India and discuss the various challenges involved in enforcing the same.

In March 2010, a vaccination trial for the Human Papilloma Virus (HPV) ground to a halt after media reports emerged of the deaths of 7 girls enrolled in the trials. Although the inquiry committee concluded that the deaths were not caused by the vaccine, it remarked on the non-adherence to ethical standards by trial organisers during the study. Specifically, the committee pointed out the failure to obtain proper informed consent, provide explanation for the role or usefulness of HPV vaccination and the inability of the Principal Investigators to monitor adverse effects during the study.
This was not the first time when a clinical research trial came into the spotlight in India for controversial reasons. In the 1970s and 80s, more than a thousand women were inducted into a study by researchers at the Institute for Cytology and Preventive Oncology in New Delhi to monitor the progress of precancerous lesions of the cervix. Women identified with these lesions were left untreated — several later developed invasive cancer.
These controversies demand focus on the ethical standards of biomedical research in our country. What are the ethical guidelines for clinical research, who formulates them and who ensures compliance of safety standards? And what happens when someone flouts these regulations? We approached Urmila Thatte, Professor and Head at the Department of Clinical Pharmacology, Seth GS Medical College& KEM Hospital, Mumbai for answers. Thatte serves on several vigilance and ethics committees as well as task forces in India. She is also on the WHO Expert Advisory Panel on Drug Evaluation, and serves as the Secretary of the Forum for Ethics Review Committees in India.
Ethical guidelines – an overview
“Currently the Indian Council of Medical Research (ICMR) is mandated with forming ethical guidelines in India,” explains Thatte, “But these are just guidelines, i.e. there is no legal status accorded to them. And although many people argue that adherence to these is not mandatory, if you go to a court of law, a violation could go against you since these are national guidelines officially released by a government agency.”
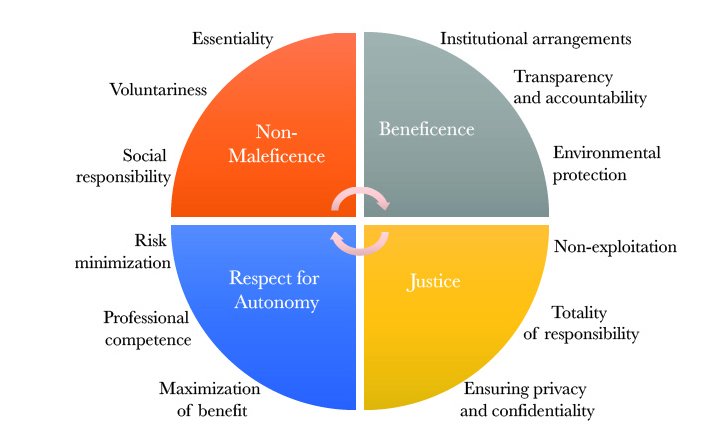
We peeked into what these guidelines are. Funded by the Government of India through the Ministry of Health & Family Welfare, ICMR is the apex body that formulates, coordinates and promotes guidelines for conducting all health-related research in India. This includes biomedical, social, genetic and behavioural studies as well as the complementary systems of AYUSH. In 2017, ICMR released revised guidelines titled “National Ethical Guidelines for Biomedical and Health Research Involving Human Participants”. These guidelines reiterate the four basic ethical principles: non-maleficence, respect for autonomy, beneficence and justice.
Expanded into 12 general principles of (i) essentiality (ii) voluntariness (iii) non-exploitation (iv) social responsibility (v) ensuring privacy and confidentiality (vi) risk minimization (vii) professional competence (viii) maximization of benefit (ix) institutional arrangements (x) transparency and accountability (xi) totality of responsibility and (xii) environmental protection, these guidelines aim to ‘protect the dignity, rights, safety and well-being of research participants’. Apart from ICMR, “Schedule Y” of the Drugs and Cosmetics Rule, and Central Drugs Standard Control Organization (CDSCO) also provide guidelines that adhere to international standards for clinical research practice.
Despite the existence of these guidelines, we do not have any substantial data on compliance (or lack thereof) by research organisations or companies. “That’s the problem,” says Thatte, “There is hardly any compliance check of the guidelines. The fact is that ICMR has released these guidelines and researchers are expected to follow them. Lack of legislation means even the evidence of violations is low. I personally don’t know the numbers, but compliance tends to be less than desired.”
Ethics Committees and Gatekeeping

In the face of limited regulatory oversight, the repercussions that a researcher, a journal or an institute might face following misconduct are limited. This is where the role of ethics committees (EC) becomes central. Most reputed journals, for example, insist on EC approval before a researcher embarks on a human clinical trial. Ethics committees require a quorum of 5 ‑15 members, having at least one each of basic biomedical scientist, clinician, legal expert/retired judge, social scientist/NGO representative, ethicist and lay person from the community. ECs are also required to undergo a methods and registration process for functioning and only thereafter get the authority to approve clinical research involving human subjects.
The entire process aims to ensure that requirements of patient confidentiality and informed consent are met. “Ethics committees ask investigators how they will protect information, and if the answers are acceptable, then that is adequate for approval,” explains Thatte, “The ECs also decide on what trials would need human data based on the objectives of the study and what previous data is being given to them. Even re-use of data requires approval, though you may not need patient consent.”
However, having ECs does not completely eliminate the issues of ethical violations in clinical research. ECs themselves have faced several challenges in the past including “inadequate formal training, contribution from non-technical members, administrative support, as well as no Standard Operating Procedures or SOPs and a heavy workload” (as Thatte described in this paper). Unlike in animal research, India does not have a central biomedical ethics committee. Thus each independent or institutional ethics committee works in isolation with their own sets of predicaments.
“They [ethics committees] have improved a lot in the last 10 – 15 years,” says Thatte, “Registration with Drugs Controller General of India (DCGI) has become mandatory, which has helped improve the structure. The DCGI office is quite vigilant and it is not easy to get registered. Which means those that are registered are of a certain standard, with SOPs in place.” Thatte shuffles through her papers, and elaborates on the numbers — about a 1000 institutional ECs were registered in 2013, out of which 615 were eligible for re-registration in 2016. The eventual number was only about 449. Eligibility criteria, which entail re-application after 3 years of first registration, have thus become more stringent.
Are we there yet?
ICMR’s other major contribution to increase transparency is the initiation of the Clinical Trials Registry of India (CTRI) in 2007. CTRI is a public domain database where researchers are expected to register their trials and upload all information related to protocols, conformity to ethical standards, and results of the trials, allowing others to freely access relevant information.
These administrative measures by government agencies have helped define the ethical boundaries in Indian clinical trials, but the buck doesn’t stop here. Increased vigilance and implementation of guidelines, such as through legal avenues, would ramp up the quality of research done in the country. It is equally important that the public becomes aware of their rights as patients in clinical trials. If a trial medicine produces side effects, a patient can report it on the free ICMR helpline (1800−180−3024) or mail pvpi.compat@gmail.com. Physicians are also expected to identify and report such adverse reactions in their patients.
There is still a long way to go. We need more clinical research that keeps the diversity of India, including varied socio-economic factors, ethnical & genetic heterogeneity in focus. Despite corrective actions being put in place in the last 5 years or so, many amendments are still ambiguous and some (such as Drugs and Cosmetics (Amendment) Bill) are yet to be passed in the parliament.
With stricter regulations in 2013 – 14, global clinical trials in India went through a deceleration phase. Agreeably thus, corrective regulatory reforms that protect patients, ensure ethical oversight and also provide positive environment for quality clinical research are the way forward.
Thank you for reading this article. Please let us know your views on this subject in the comments below.